we were complete INTRODUCTION and OCCURRENCE OF LANTHANOIDS in previous blog. now its turn to have a look on ELECTRONIC CONFIGURATION AND POSITION OF LANTHANOIDS IN THE PERIODIC TABLE.
Lanthanoids involve in the gradual filling of 4 f-orbitals. The ground state electronic configuration of each lanthanoid element has two electron in 6 s-orbitals. Sincs the energies of 6s,5d and, 4f are nearly equal, therefore, the order of filling of the 4 f-orbitals in atoms shows some irregularities. At lanthanum (Z=57), both 5d and 4f orbitals become stable due to the increased effective nuclear charge because of poor shielding effect of 6s electrons .It is, therefore, observed that a lanthanum, the comming electron enters the 5 d-orbital and electronic configuration of lanthanum is
La (Z=57) : [Xe]⁵⁴4f ⁰ 5d¹ 6s²
However on moving further after the lanthanum, the energy of 4f-subshell becomes distinctly lower than the 5d-subshell and therefore the next electron in cerium enters 4f-subshell. The electronic configuration of cerium therefore, is represented as
Ce (Z=58) : [Xe]⁵⁴ 4f ² 5d⁰ 6s²
This trend of filling 4f-subshell continues further until we reach ytterbium in which 4f-subshell gets completely filled. thus, ytterbium has the electronic configuration
Yb(Z=70) : [Xe]⁵⁴ 4f¹⁴ 5d⁰ 6s²
Now, after filling 6s– and 4f– orbitals, the next electron does not have a choice and has to go to 5d-orbital. So, in lutetium (Z=71), the next electron enters 5d-orbital and its electronic configuration is
Lu (Z=71) : [Xe]⁵⁴ 4f¹⁴ 5d¹ 6s²
It may be noted here that the electronic configuration of penultimate shell (n=5) is invariably s²p⁶ except for three elements, namely, lanthanum ( Z=57 ), gadolinium ( Z= 64) and lutetium (Z = 71) in which these have one electron in 5d-subshell also. It may also be observed that 4f-orbitals are completely unoccupied in case of lanthanum ( Z = 57) , are half filled in case of europium ( Z= 63), and gadolinium (Z=64) and completely filled in lutetium ( Z = 71).
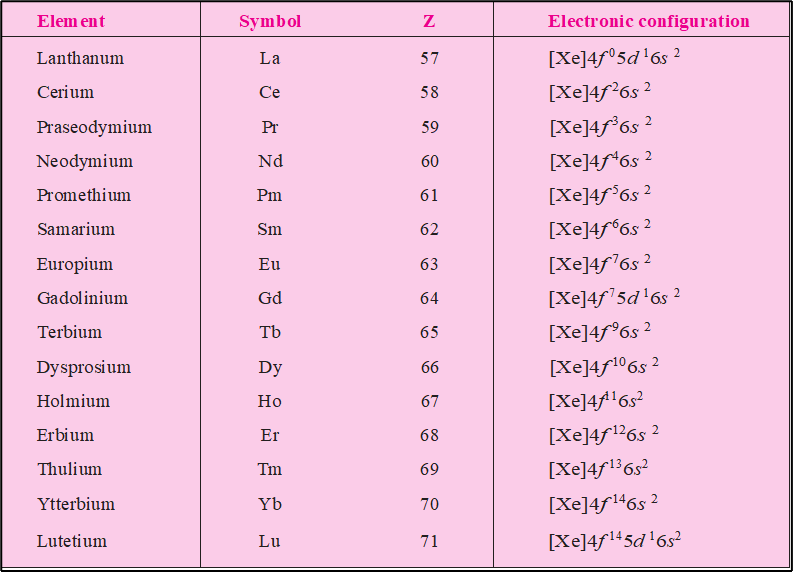
Image reference : flexiprep.